Abstract
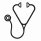
Familial erythrocytosis (congenital erythrocytosis, FE), is a rare congenital disorder defined by elevated hemoglobin and hematocrit, with different genetic background. Clinically, FE is difficult to distinguish from polycythemia vera(PV).
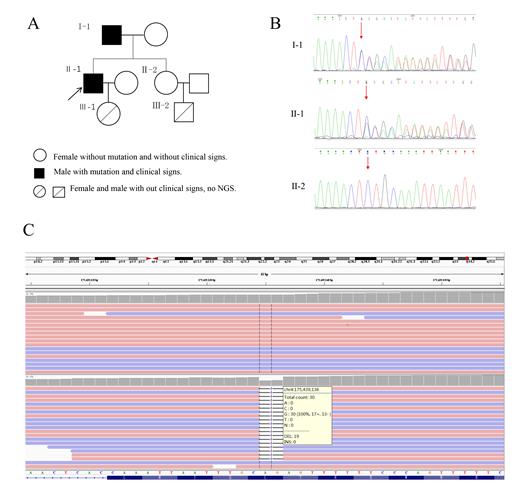
A 53-years-old male was diagnosed with polycythemia vera without discovery of the JAK2 mutation when he was 31-years-old. The patient's family history revealed his father, a 86-years-old male, also suffered polycythemia for more than 20 years. The pedigree of the Chinese family with erythrocytosis is shown in Figure 1a. The index patient was a 53-years-old male (patient Ⅱ-1, Fig.1), who was hospitalized in our department in 2019 with a 22-year history of elevated red cell mass (RCM). When he was 31-years-old, he initially diagnosed with polycythemia vera without discovery of the JAK2 mutation. Over the last two decades he had irregular phlebotomy almost every two years and seldom prescribed any cytoreductive treatment. At our department he accepted 2 venesections because of Hct level of 64%. Upon medical history taking he reported that his father had suffered by polycythemia with more than 20 years, and were undergoing occasional phlebotomies.The father of the index patient was a 86-years-old male (patient Ⅰ-1, Fig.1), who was diagnosed with polycythemia for more than 20 years and suffered from diabetes. Similar to his son, he did not use any cytoreductive agent, and he had been phlebotomized occasionally. He was chronically treated with low-dose of aspirin . In our department he was treated with erythrocyte separation because of Hct level of 58.9%. He did not report any thrombembolic event. For the last one year of follow-up, the patient continued taking aspirin and her Hct level fluctuated between 54% and 57% while rejected to receive erythrocyte separation again.The elder sister (subject Ⅱ-2 Fig.1) of the index patient did not have any clinical and laboratory signs of elevated RCM as of January 2019, she was a 57-years-old female, who suffered from hypertension and diabetes.The daughter (subject Ⅲ-1 Fig.1) and the nephew (subject Ⅲ-2 Fig.1) of the index patient also did not have any clinical and laboratory signs of elevated RCM as of January 2019. They were subjected to whole exome sequencing (WES), the results revealed four mutations in all three of them, among, a frameshift mutation in the 15-hydroxyprostaglandin dehydrogenase(HPGD) gene is contained in both father and son, which at position c.310_311 ,translating into c.310_311delCT nucleotide mutation and p.L104Afs*3 amino acid mutation. In order to verify the mutation, we adopt the method of Sanger sequencing, then confirmed the presence of the same HPGD frameshift mutation in the index patient and his father. However, The mutation was absent in the elder sister of the index patient, when she was examined by WES and Sanger sequence. The ARHGAP26 mutation is contained in all three of them, but is a type of somatic mutation. The two mutations of VHL and FANCD2 are inexistent in the index patient, but are contained in his father and his elder sister.
In conclusion, here we reported the first extensive genetic and clinical study of a family with two members carrying the HPGD gene frameshift mutation. Although functional studies were not made to confirm the pathogenic role of this mutation, the type and location of the mutation suggest that it can be the cause of the erythrocytosis observed in two patients. This study demonstrated the utility of the WES/NGS as the tool for identification of mutations in congenital erythrocytosis as well as helps to discovery these rare erythrocytosis-associated genes. The role and pathogenesis in haematopathy of HPGD mutation has been seriously underestimated, which is deserved to be explored in depth.
Acknowledgment:The research was supported by the Public Technology Application Research Program of Zhejiang, China (LGF21H080003), the Key Project of Jinhua Science and Technology Plan, China (2020XG-29 and 2020-3-011), the Academician Workstation of the Fourth Affiliated Hospital of the Zhejiang University School of Medicine (2019-2024), the Key Medical Discipline of Yiwu, China (Hematology, 2018-2020) and the Key Medical Discipline of Jinhua, China (Hematology, 2019-2021).
Correspondence to: Dr Jian Huang, Department of Hematology, The Fourth Affiliated Hospital of Zhejiang University School of Medicine. N1 Shangcheng Road. Yiwu, Zhejiang, Peoples R China.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract